Let’s start by defining quality in simple terms and with approach of continuous improvement. Quality is providing our customers the product/service they expect (i.e., meeting customer´s expectations).
In this sense, then, continuous improvement is delivering our customers a better product than they are waiting (exceeding customer expectations).
Pharmaceutical is one of the most supervised and regulated industries all over the world, through national governmental organisms, like US’ FDA, UK’s MHRA or Australia’s TGA, as well as international organizations: WHO, EU’s European Medicines Agency (EMA), or US-EU-Japan International Conference of Harmonization (ICH).
These organizations focus on keeping up-to-date and monitoring compliance of pharmaceutical guidelines, which have the status of law in all the countries. In the pharmaceutical slang, these guidelines are known as the Good Manufacturing Practices (GMPs), which are defined as a set of guidelines and related activities, aimed at ensuring that drug products have and maintain the identity, purity, concentration, power and safety required for their use.
GMPs have been created and are being updated over the years, learning from the mistakes and refining the methods, procedures and general policies for drug products manufacturing.
We constantly hear in the news (or watch documentaries) about the wonders of the technology applied in medicine and drug products (awesome! Aren’t they?). However, for mass manufacturing, pharma industry faces a paradox: even with the introduction of futuristic drugs, it has manufacturing and control processes techniques of that would even be considered obsoletes.
Up to some years ago, regulatory agencies, with the aim of protecting safety of patients, seemed to give more importance to the manufacture of drug products that strictly meet quality specifications through a system of trial-and-error, instead of looking for the latest trends in manufacturing.
Meanwhile other industries, manufacturing lines and processes are constantly evaluated to find improvements, strict regulations to produce drugs may slow down improvements in pharma industry: as part of the approval process of a drug, factories must go through rigorous inspections of regulatory agencies.
The same applies to a new drug: before being approved it for going to the market, regulatory authorities should exhaustively review a dossier which contains the information which demonstrate the drug product is safe and is batch-to-batch consistently manufactured.
After approval, some potential changes in a production process, must be evaluated by regulatory bodies before going for it, which involves time and bureaucratic paperwork. Eventually, this process discourages the update of companies due to delays in production, which carries out economic consequences.
Even with the efforts to focus on quality, pharma has failed to stay at the same level with other industries in terms of efficiency and productivity, largely by the cost and work involved in revalidation for processes improvement. In 2005, it was estimated that percentage of rework and annual rejections in US pharma factories was between 5-10% (USD $4.5 – $9 billion). On the other hand, semiconductor industry had a percentage of 0.0001%.
For new drug products, ICH’s Q8 guideline establishes basis to developments be carried out within a design space, in such way modifications after health authority’s approval lasts less time at the same time product’s quality is kept and assured.
But, what happens with products that already exist? Which methods and tools allow us to optimize them?
To optimize processes and resources in the pharmaceutical industry, it is needed to implement systems or methodologies that allow us:
- Identifying improvement opportunities and their potential benefits.
- Determining causes that lead to the current situation and possible opportunity areas.
- Defining the variables that may impact on the process and the extent they do.
- Running experimental trials to control or optimize variables.
- Defining measures that allow us to maintain consistently trough time achieved improvements.
- Keeping documented evidence of each improvement.
Now, how can we use Lean-Sigma in the pharmaceutical industry?
As we know, Lean-Sigma’s philosophy is the combination of two methodologies of continuous improvement:
- Lean manufacturing: improving productivity and eliminating waste by mapping processes and identifying improvement areas.
- Six Sigma: improving quality and reducing the level of defects by statistical analysis and statistical in-process control.
Methodology used in lean sigma, known as DMAIC, and that can be implemented in any industry or activity for continuous improvement, can be outlined in following figure:
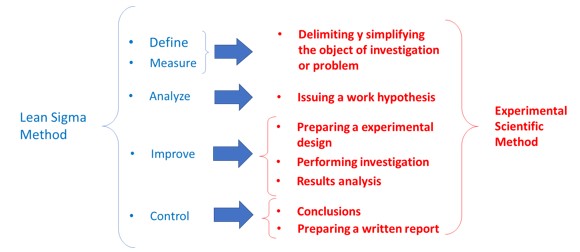
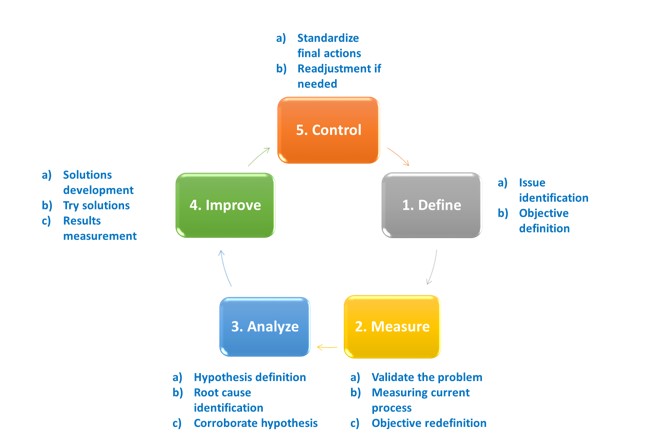 It is easy to ask; how can we relate a methodology of “universal” application to an industry that bases its development and manufacturing main activities on scientific basis? Well, lean-sigma methodology has much in common with the experimental scientific method, as we can see in the following figure:
It is easy to ask; how can we relate a methodology of “universal” application to an industry that bases its development and manufacturing main activities on scientific basis? Well, lean-sigma methodology has much in common with the experimental scientific method, as we can see in the following figure:
In our next article, we will discuss an example of productivity improvement in a pharma manufacturing process by applying lean-sigma methodology.
